BEFuel: Sustainable Production of Valuable Substances from Wastewater and Exhaust Gases
Contact: Beyzanur Cicec, Dirk Tischler
Cooperation: Ruhr-Universität Bochum | Chair of Urban Water Management and Environmental Engineering & Inorganic Chemistry; SolarSpring GmbH; Emschergenossenschaft Lippeverband (EGLV); Institut für Automation und Kommunikation e.V. (ifak); Fraunhofer UMSICHT
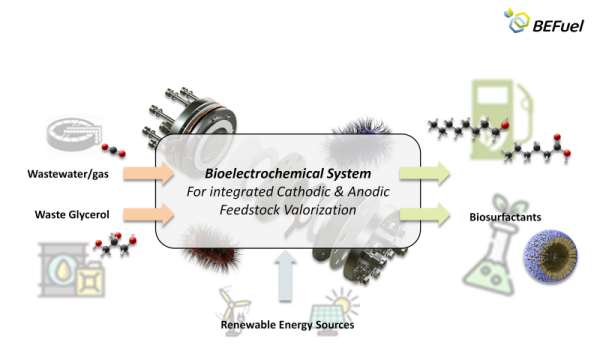
The BEFuel joint project (“Gekoppelte bioelektrochemische Produktion von E-Fuels und hochwertigen Chemikalien aus Abgasen und Abwässern”) aims to generate new valuable substances from wastewater and exhaust gases. The bioelectrochemical system enables the production of organic C6 and C8 acids, which serve as starting materials for biodiesel and biogas.
By fixing CO2 and utilizing green hydrogen, microorganisms can produce high-quality chemicals. The green hydrogen is generated through the electrolysis of wastewater from a sewage treatment plant. At the anode of the electrolyzer, crude glycerol, a byproduct of biodiesel production, is used. The oxidation products of glycerol are utilized by other microorganisms to form biosurfactants.
Funding: The BEFuel project is funded by the Federal Ministry of Research, Technology and Space (BMFTR).
Press release: https://www.umsicht.fraunhofer.de/de/presse-medien/pressemitteilungen/2024/industrielle-biooekonomie.html
Establishing a Pan-European Network on Computational Redesign of Enzymes (COZYME)
Contact: Dirk Tischler

The COZYME (COmputationally assisted design of enZYMEs) Action comprises a Pan-European collaborative network aimed at developing and implementing state-of-the-art computational tools for rapid enzyme improvement. This will solve a key bottleneck in biotechnology: the exploitation of industrially relevant enzymes. Specifically, the Action focuses on three issues:
1. Improvement of generic enzyme properties such as stability and solubility;
2. Optimization of catalytic properties e.g. activity and stereoselectivity;
3. Advancement of experimental approaches to generate and evaluate computational predictions;
These goals are intended to be of particular benefit in the context of training young researchers in the development and use of computational methods in biotechnology.
Duration: October 2022 – October 2026
Funding: European Cooperation in Science and Technology (COST)
Link: https://www.cost.eu/actions/CA21162/
BiodeCCodiNNg
DC 8 - Towards N-N bond formation via N-hydroxylation in a biocatalytic cascade
Contact: Dirk Tischler

From enzyme discovery to industrial implementation – creating novel biocatalysts for industrial and pharmaceutical biotechnology; these are the focal points in our EU funded doctoral network. Herein, we combine basic research and applied engineering to deliver new synthetic routes to chemically relevant products in more efficient and cleaner ways than the present ones. The overall project connects three work packages (WP1: Enzyme discovery and characterization of novel NN- and CCzymes; WP2: Structure elucidation and enzyme engineering towards novel reactivities; and WP3: Reaction engineering and biocatalytic applications) which are investigated both at research institutes as well as at industrial sites.
Our part is important for the NN-coupling enzymes as we focus on the activation of amine groups by means of N-hydroxylating enzymes. Along this we implement methods to describe enzymes from phylogenetic point of view, to uncover mechanistic details, to evolve them by rational design, and to stabilize enzymes by immobilizations techniques.
Funding: The doctoral network is funded in course of the MARIE SKŁODOWSKA-CURIE ACTIONS (ID: 101073065)
Link: https://biodeccodinng.eu/
Glutathione in degradative metabolism of Actinobacteria
Contact: Max Scholz, Dirk Tischler
The Actinobacterium Gordonia rubripertincta CWB2 has a novel styrene degradative pathway comprising a glutathione dependent step. In course of this project we study the novel enzymes from strain CWB2: glutathione S-transferase and glutathione reductase. Here, general cloning and protein production methods are used in combination with sophisticated UHPLC and LC-MS methods to characterize enzyme activities. Furthermore, we employ mutagenesis strategies to knockout selected genes in strain CWB2 or to bring others under selected regulators to allow gene silencing as well as expression in those Actinobacteria.
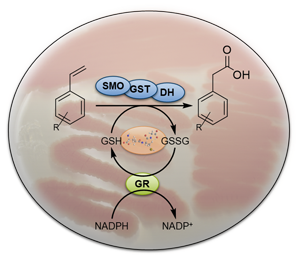
Funding: The project is supported by the DFG-funded Research Training Group MiCon (RTG 2341). In addition, we get support for the project by Mercator Research Center Ruhr (An-2018-0044).
Biotransformation of lignin-monomers by flavin-dependent oxidases
Contact: Nils Weindorf, Tobias Rapsch, Dirk Tischler
Cooperation: Willem J.H. van Berkel, Wageningen University; Andrea Mattevi, University of Pavia
Flavin-dependent oxidases play a central role in biocatalysis but often have limitations in stability and substrate specificity. Through homology modeling, we aim to identify new enzymes with an expanded substrate spectrum. Additionally, we are working on optimizing suitable enzymes by combining classical enzyme kinetics with AI-supported structure predictions. Based on these data, targeted mutations will be performed to improve activity, stability, and substrate breadth through enzyme engineering. The optimized biocatalysts will then be used in multi-enzyme cascades, preferably immobilized in cell-free systems for the efficient conversion of industrially relevant substrates.
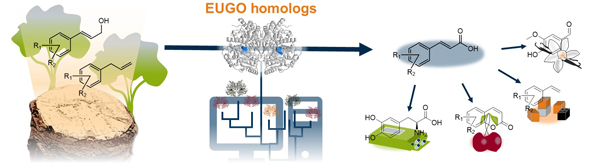
Funding: The project is funded by a doctoral fellowship from the German Federal Environmental Foundation (DBU) for Nils Weindorf. Tobias Rapsch is funded by the German Research Foundation (DFG).
![]()
Contact: Carolin Mügge
The project BioCOnversion unites multidisciplinary expertise from academia and industry in a cross-border consortium to make CO-containing process gases available for the production of added-value chemicals. The German Federal Ministry of Education and Research (BMBF) funds this innovative bioprocess to convert syngas into a defined plastic precursor by evaluating different technology approaches.
Carbon monoxide (CO)-containing process gases, abundant in the BIG-Cluster region through numerous industrial sites, can be valuable feedstock streams for the biotechnical production of building blocks that are currently produced via petrochemical process routes. Mid-chain carbon compounds with multifunctional groups are of special industrial interest. Since they are conventionally generated from fossil resources, routes using renewable non-food feedstocks to provide such precursors would be a major step to establish a sustainable economy. Therefore, BioCOnversion aims at developing and implementing a sustainable process from CO to a defined polymer precursor by evaluating different technologies. An international consortium of industrial and academic partners join their high-level, multidisciplinary expertise to develop a process comprising the primary conversion of CO/syngas into an intermediate through gas fermentation and its enzymatic upgrading conversion to a defined plastic precursor.
RUB's part of the project focuses on enzymatic uprading of the intermediate produced by syngas-converting microorganisms. Within this, the full spectrum of strain and enzyme optimization as well as reaction tuning is tackled. The project is corroborated by mechanistic studies on the target enzymes.
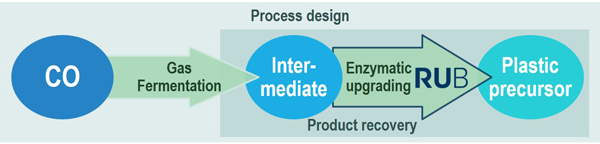
external links: https://www.bigc-initiative.eu/bioconversion.php
Press release: pdf
Funding: The project is funded by the BMBF (03INT513BF) in frame of the BIG-cluster initiative.
ChemBioCat
Contact: Carolin Mügge, Dirk Tischler
Cooperation: Prof. Dr. Pablo Sobrado, Virginia Tech, Blacksburg
The project aims to establish novel chemo-enzymatic cascades towards valuable building blocks and can be divided into two subprojects:
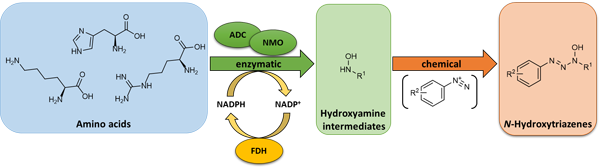
Hydroxytriazenes and hydroxytriazoles are important fine chemicals with biological activity and those can be tested to affect bacteria, fungi or single enzymes.
Funding: The project is supported by the State North Rhine-Westphalia funding the Research Group Grant (PtJ-TRI/1411ng006).
Degradation industrially relevant azo dyes
Contact: Anna Christina Ngo, Selvapravin Kumaran, Dirk Tischler
Cooperation: Dr. Isabel Bento, EMBL Hamburg
In this project we use either isolated enzymes or novel bacterial isolates to convert azo dyes. With respect to the enzymes we employ various flavin-dependent and -independent azoreductases to break the azo bridge leading to various aromatic amines. Here the prototype enzyme is AzoRo from Rhodococcus opacus 1CP which is subject to protein engineering and structural investigations. In addition, we screen for bacteria with the capability to degrade various azo dyes or even use them as sole source of carbon. Here we employ methods of functional genome annotation and biodegradation techniques in combination with chromatographical analytics.
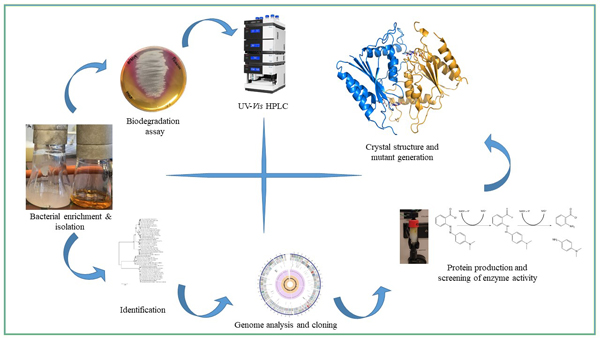
Funding: The project is supported by a KAAD predoctoral scholarship awarded to Anna Christina Ngo.
EnzymPrint
Contact: Dirk Tischler
Cooperation: Hirsch Engineering Solutions GmbH & Co. KG, Eichstätt
The project aims at immobilizing enzymes on biocompatible materials to provide access towards cascades. Exemplarily, will be the synthesis of trehalsoe from glucose-moieties and polyphosphate investigated.

external links: https://www.hirsch-es.de/
Funding: The project is funded as a cooperative project by the German Federal Ministry for Economic Affairs and Energy (KK5161101CS0).